2242020 His theory contained five main propositions. The postulates and limitations of Daltons atomic theory are listed below.

English For Chemistry Video English Narration
This later became known as Daltons atomic theory.

4 ideas in dalton's theory. Atoms cannot be subdivided created or destroyed. Google Classroom Facebook Twitter. John Dalton-English chemist that proposed first atomic theory in 1803.
For example in 1897 English physicist J. Dalton Atomic Theory fails to explain the existence of allotropes. Daltons theory explained many principals of chemistry with simplicity.
All atoms of a particular element share identical properties including weight. Was John Daltons theory accepted. Matter is composed of exceedingly small particles called atoms.
3 Compounds are formed by a combination of two or more different kinds of atoms. Atoms of different elements contain different mass. 2 All atoms of a given element are identical in mass and properties.
All elements are composed of indivisible particles. When excited by means of an electrical current atoms break down into two parts. The general tenets of this theory are as follows.
Thomson 18561940 discovered that atoms are not indivisible. Many of Daltons assumptions have been changed but they still keep the main ideas that he proposed long ago. John Dalton and the Scientific Method.
The postulates of Daltons atomic theory. 8242019 First published in 1807 many of Daltons hypotheses about the microscopic features of matter are still valid in modern atomic theory. In 1803 Dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas.
Atoms are indivisible and indestructible. All Matter Is Composed of very tiny partlcles called atoms. Atoms are indivisible and indestructible.
2 - Atoms combine in simple whole number ratios to form compounds. Click again to see term. Here are the postulates of Daltons atomic theory.
All matter is composed of. As each part of Daltons theory was tested new ideas about atoms were developed through experimentation and new models were proposed and tested. 3172021 Daltons Atomic Theory 1804 From his own experiments and observations as well as the work of his peers Dalton proposed a new theory of the atom.
Daltons Atomic Theory also suggested that an atom is the smallest part of an atom that can take part in a chemical reaction. A chemical reaction is a rearrangement of atoms. Tap card to see definition.
An atom is the smallest unit of an element that can participate in a chemical change. Which points do we still use today and what have we learned since Dalton. Matter is composed of exceedingly small particles called atoms.
Consequently what were the main. Here are the postulates of Daltons atomic theory. Tap again to see term.
This is the. 3 - Each element is composed of tiny indestructible particles called atoms. First published in 1807 many of Daltons hypotheses about the microscopic features of matter are still valid in modern atomic theory.
Atoms of the same element are exactly alike. 4112007 Daltons theory can be called modern because it contained statements about atoms that could be tested experimentally. An atom is the smallest unit of an element that can participate in a chemical change.
As each part of Daltons theory was tested new ideas about atoms were discovered. First published in 1807 many of Daltons hypotheses about the microscopic features of matter are still valid in modern atomic theory. An atom is the smallest unit of an element that can participate in a chemical change.
1- Atoms of one element cannot change into atoms of another element. Matter is composed of exceedingly small particles called atoms. Atoms of different elements combine in fixed whole-number ratios when.
This implies that the Dalton atomic theory fails to explain the differences in properties of charcoal graphite and diamond allotropes of carbon. Daltons theory had five major parts. His theorys value lies in the research ideas it contained.
One may also ask what are 3 main ideas in Daltons atomic theory. It stated that all matter was made up of small indivisible particles known as atoms. Here are the postulates of Daltons atomic theory.
All matter is composed of extremely small particles called atoms. 12192015 Daltons atomic theory was a scientific theory on the nature of matter put forward by the English physicist and chemist John Dalton in the year 1808. All matter is comprised of tiny definite particles called atoms.
Click card to see definition. Changes In Daltons Atomic Theory Over the years scientists have made many advances in finding a more accurate version of Daltons Atomic Theory. 1 All matter is made of atoms.

Chapter 4 Partial Structure Of The Atom Ppt Download

John Dalton His Theory Of The Atom Dalton S Model Of The Atom Ppt Download

Main Ideas 1 Atom 2 Dalton S Atomic Theory Key Concepts 1 How Did Democritus Describe Atoms 2 How Did John Dalton Further Democritus S Ideas On Atoms Ppt Download
Chapter 4 Partial Structure Of The Atom Ppt Download

Dalton S Atomic Theory Postulates Limitations Merits Chemistrygod

Main Ideas 1 Atom 2 Dalton S Atomic Theory Key Concepts 1 How Did Democritus Describe Atoms 2 How Did John Dalton Further Democritus S Ideas On Atoms Ppt Download
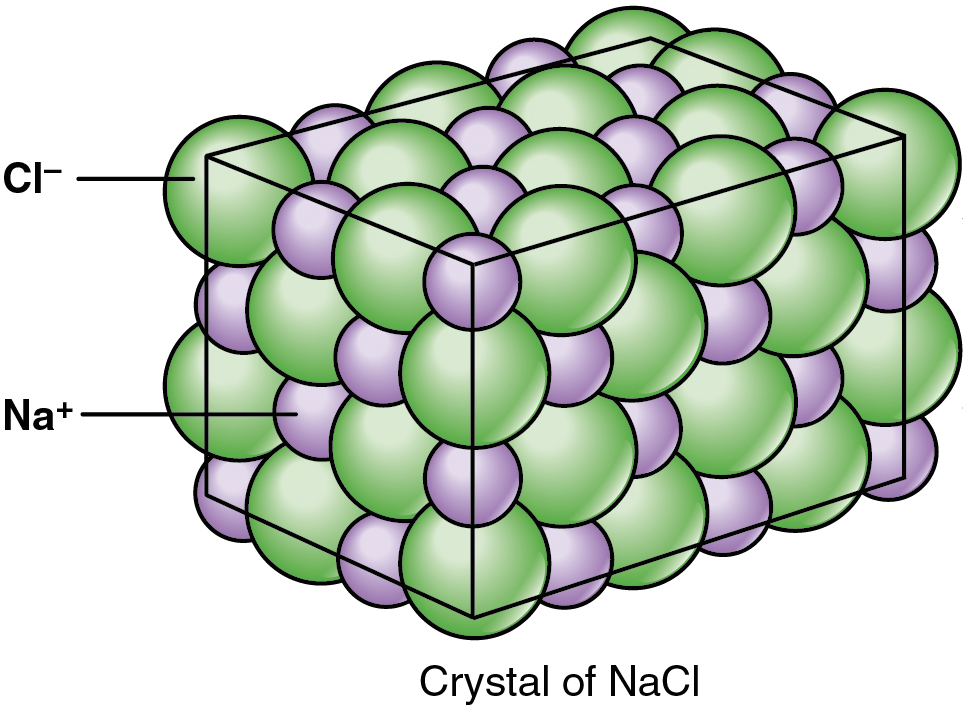
Dalton S Atomic Theory Article Khan Academy

Dalton S Atomic Theory Atomic Theory John Dalton Atomic Theory Chemistry

Post a Comment
Post a Comment